The effectiveness of virtual reality
based pain control with multiple treatments.
HUNTER
G. HOFFMANac, DAVID R. PATTERSONb,
GRETCHEN
J. CARROUGHERb, and SAM R. SHARARd
aHuman
Interface Technology Laboratory, University of Washington
bDepartment
of Rehabilitation Medicine, University of Washington School of Medicine
cDepartment
of Psychology, University of Washington
dDepartment
of Anesthesiology, University of Washington School of Medicine
Hoffman,
H.G., Patterson, D.R., Carrougher, G.J., &
Sharar, S. (2001). The
effectiveness of virtual reality based pain control with multiple treatments.
Clinical Journal of Pain, 17, 229-235.
Abstract:
Objective: The present study explored whether immersive virtual reality continues to reduce pain (via distraction) with repeated use.
Setting: Burn care unit at a regional trauma center.
Patients: Seven patients aged 9-32 years, mean age = 21.9 years (average of 23.7% total body surface area burned, range TBSA = 3% to 60%), performed range of motion exercises of their injured extremity under an occupational therapist’s direction on at least three separate days each.
Intervention: For each physical therapy session, each patient spent equal amounts of time in VR and in the control condition (no distraction). The mean duration of physical therapy in VR was 3.5, 4.9 and 6.4 minutes for the first, second and third session, respectively. Condition order was randomized and counterbalanced.
Outcome measures: For each of the three physical therapy sessions, five visual analog pain scores for each treatment condition served as the dependent variables.
Results: Pain ratings were statistically lower when patients were in VR, and the magnitude of VR pain reduction did not diminish with repeated use of VR. The results of this study may be examined in more detail at www.vrpain.com.
Conclusions: Although the small sample size limits generalizability, results provide converging preliminary evidence that virtual reality can function as a strong nonpharmacologic pain reduction technique for burn patients during physical therapy. Results suggest that virtual reality does not diminish in analgesic effectiveness with three (and possibly more) uses. Virtual reality may also have analgesic potential for other painful procedures or pain populations. Practical implications are discussed.
Keywords: Burn pain-Virtual reality-Distraction-Analgesia.
The effectiveness of virtual reality based pain control with multiple treatments: A controlled study.
Successful participation in physical therapy after a severe burn injury is often crucial for minimizing long-term disability. Without physical therapy, the normal healing process in severely burned and grafted skin results in heterotopic scarring, and severe contractures1. Aggressive physical therapy increases the flexibility/elasticity of healing skin, and helps maintain normal range of motion and function1. Unfortunately, the pain experienced during therapeutic movement of burned, grafted, and healing extremities can discourage patients from performing their exercises2. Patient non-adherance to such exercises can lead to additional surgery (e.g., more skin grafts) or permanent reduction in limb mobility1.
Opioid analgesics have long been considered the “gold standard” of pharmacologic analgesics3. Although such drugs form the cornerstone for nearly any burn pain management plan4,5, side effects limit their use (e.g., nausea6, vomitting6, constipation6, sedation6, itchiness6, urinary retention6, cognitive impairment6, hallucinations6, delerium6, respiratory depression6, tolerance7, and risks for physical and psychological dependence8). These side effects can become especially problematic when opioid analgesics are administered over prolonged periods. An additional concern about opioid analgesics is that, even though they represent the best approach to burn pain, and are highly effective for treating background pain, their analgesic efficacy for extreme procedural pain is limited. Patients with severe burns routinely experience severe pain during wound care, despite aggressive pain control with potent opioid analgesics9,10,11. In one study of patients with severe burns, 84% of the patients given a typical dose of morphine still reported severe to excruciating pain during wound care9. Two thirds of the burn patients in that study rated their worst pain during wound care as “excruciating” 9.
As a result of the
strong psychological component to pain perception, supplemental use of
non-pharmacologic analgesic techniques can be effective, e.g., mental imagery12,
watching a video13, biofeedback14, enhanced control15,
parental participation16 and hypnosis17,18,19. Cognitive/behavioral strategies have been
found to be useful for a wide variety of pain etiologies, and significantly
reduced pain reports in 85% of 47 studies (meta-analysis20).
Distraction is a cognitive-behavioral intervention particularly useful with
burn pain.5,13 Immersive virtual reality is an attention-grabbing
illusory reality created in the mind of the VR user/patient. Researchers argue that virtual reality (VR)
may be an unusually effective distraction21. Performing a virtual
reality task draws heavily upon conscious attention22,23 leaving
less of this cognitive resource to devote to pain perception. With less attention available for evaluating
nociceptive input, patients subjectively experience less pain.24 The
convergence of multi-sensory input (sight, sound, and sometimes touch) in the
virtual environment creates a sense of "presence" in the environment
(i.e., the illusion of going into the computer generated world). In this respect, immersive VR differs from
more simple forms of distraction (e.g.,
video movies, interactive video games) by increasing the amount of the
patient’s attention drawn into the virtual environment.25 A second
mechanism by which VR may improve analgesia is through the reduction of visual
cues associated with the painful procedure.
Children often develop strong conditioned anxiety responses to visual
cues associated with their wound care or rehabilitation procedure.26 Anxiety-inducing sights and sounds of the
hospital or clinic environment that likely exacerbate the patient’s pain are
blocked out by the VR helmet worn by patients during the procedure, thereby
limiting these negative cues and aversive conditioning. Although the majority of patients fixate
most of their attention on the wound care during conventional treatment,
previous studies suggest that patients are able to shift their attention away
from their pain with VR21,27.
Researchers measured the pain levels of two pre-adult patients undergoing staple removal from skin grafts while being distracted by VR for three minutes and staple removal while playing Nintendo64TM for three minutes (order counterbalanced) during a single wound care session21. Patients showed the predicted drop in pain when in VR compared to the video game condition. More recently, researchers conducted a within-subject clinical study with twelve burn patients during physical therapy27. All patients reported experiencing less pain in virtual reality compared to no distraction during a single physical therapy session, and the magnitude of pain reduction from VR was statistically significant. Patients also reported large reductions in the amount of time they spent thinking about their pain during the three-minute sessions (e.g., on a 100 mm scale, “time spent thinking about pain” during physical therapy dropped from 60 mm with no VR to 14 mm with VR).
The average sized burn requiring inpatient hospitalization might require at least a week of hospitalization and numerous wound care and exercise sessions. In the case of an extremely severe burn, physical therapy sessions may take place over a period of months and number in the hundreds. In all multi-participant studies to date, each participant used VR only one time. If the analgesic effects of VR stem primarily from the novelty of this technological approach, pain control would likely become less effective with repeated use. VR pain control would be of limited value if it only worked the first time it was applied. Encouragingly, in a recent pilot case study on a single burn patient, the amount of VR-based pain reduction did not diminish with repeated treatments over a one-week period28. The present study further addresses this issue. Using a within-subjects design, we compared the efficacy of immersive virtual reality to a no distraction condition (conventional treatment) during at least three separate therapy sessions with multiple patients. We hypothesized that 1) VR would result in less pain, and less time thinking about pain than equivalent periods of physical therapy using a standard treatment protocol (no distraction), and 2) the amount of pain reduction would not decrease with repeated use.
Method.
Subjects. Seven patients with severe burn injuries participated (age range = 9-32 years, mean age = 21.9 years, mean TBSA burn size = 23.7%, range of TBSA = 3% to 60%). Patients were all hospitalized at a major regional burn facility, and all reported prior trouble tolerating their pain during physical therapy. Specifically, prior to recruitment, all patients verbally rated having a worst pain during physical therapy of 5 or higher on a visual analog scale from 0 to 10 where 0 = “no pain at all” and 10 = “worst pain.”
Potential patients were recruited by a research nurse who was in contact with occupational therapists about potential enrollees. Six of the patients were male, one female. Each patient participated in the study as many times as possible before they were released from the hospital or went to surgery. Every patient informed of the study agreed to participate, and after each physical therapy session, each patient agreed to return for more VR treatment the following day. Each patient was treated on at least 3 separate days, and only used VR during the physical therapy sessions (none had previous experience using VR). The information presented to patients at time of recruitment is shown in Appendix A.
Standard pharmacologic analgesia was administered to
patients at the discretion of the physicians and nurses for treatment of pain
and was not affected by participation in this study. Some patients’ physical therapy sessions followed shortly after
their daily dressing changes. In these
cases, the patients may still have experienced additional pharmacologic
analgesia from short-acting opioids administered to help reduce procedural pain
during their morning dressing/bandage change.
Use of a within-subject design insured that drug dosages were the same
in the VR and control condition for each individual patient. The therapist
chose the injured extremity that was either the most painful or most
troublesome (with regard to range of motion) for the patient. Each patient spent a pre-determined amount
of time performing physical therapy in VR and an equal amount of time
performing physical therapy with no VR (conventional treatment) during the same
session. The same active-assisted range of motion exercises were performed
during both experimental conditions (e.g., same number of repetitions, same
exercises performed in the same plane, stretch held for the same number of
seconds). The duration of exercise
treatment was set prior to beginning physical therapy on any given day. The order in which the treatments were
administered was randomized and counterbalanced such that each treatment
condition had an equal chance of occurring first or second for each
patient. If VR was (by chance) first
for session 1, “no VR” was first for session 2, etc.
At the end of each treatment period, maximum range of motion (ROM) of the relevant limb was measured by the occupational therapist, using a goniometer. Each patient’s ROM was measured only once per condition. Pain, the primary dependent variable, was measured immediately after each experimental treatment, during a brief (approximately 2 minute) pause in therapy. At each pause (once after therapy with VR, and once after therapy with no distraction), patients completed five retrospective subjective pain ratings using 100 mm Visual Analog Scales (i.e., VAS29,30). With respect to the last 3 minutes of physical therapy during that study condition, patients rated A) how much time they spent thinking about their pain and/or burn wound (endpoints labeled zero minutes, the entire time), B) how UNPLEASANT was physical therapy (not at all unpleasant, the most unpleasant), C) how much their wound BOTHERED them (not at all bothersome, the most bothersome), D) their WORST PAIN (no pain, worst pain), E) their AVERAGE PAIN (no pain, worst pain). The pain experience has at least two components that are separately measurable and sometimes differentially influenced31,32; a sensory component (worst pain and average pain in this study) and an affective component (unpleasant and bothersome in this study). Time spent thinking about pain is a recently reported measure of procedural burn pain.21 After filling out their pain ratings, patients in the VR condition were asked the following ratings using visual analog scales: 1) To what extent (if at all) did you feel nausea as a result of experiencing VR? (none, very much), 2) While experiencing VR, to what extent did you feel like you went into the virtual world? (I did not feel like I went into the virtual world at all, I went completely into the virtual world), 3) How real did the objects in the virtual world seem to you (completely fake, indistinguishable from a real object). Hendrix and Barfield33 describe several studies showing the reliability of a similar subjective measure of presence.
Procedure.
A Silicon Graphics Octane MXE with Octane Channel Option (www.sgi.com) was coupled with a V8 VR helmet (www.virtualresearch.com) to create an immersive, 3-D, interactive, computer-simulated environment. Eyepieces on the helmet were circular and had 60 degree diagonal field-of-view per eye. A Polhemus FastrakTM motion sensing system (www.Polhemus.com) with 6df sensors was used to measure the position of the user’s head. The first patient in the present study explored the virtual environment SpiderWorld (see Hoffman et al,28 for a detailed description) while the last six explored SnowWorld. SpiderWorld was complete with countertops, a window, and 3-D cabinets. The patient could “pick up” virtual objects with his cyberhand. For example, there was a grab bag of over 20 virtual objects on the counter, which the patient could pull out one by one and identify. Using tactile augmentation34,35 if willing, the patient could “physically” touch the furry body of a virtual Guyana bird-eating tarantula with wiggling legs, and could physically eat a virtual candy bar linked via a position sensor attached to the candy bar’s real world twin. The patient dropped a virtual spider out of a “spider bucket” with sound effects, and herded the animated spider into a sink, filled the sink with water, and turned on the virtual garbage disposal. The other six patients in the present study had the illusion of flying through SnowWorld, a virtual environment created with CreatorTM modelling software and VEGATM development software from www.MultiGen.com. SnowWorld depicts an icy 3-D virtual canyon with a river and waterfalls. Patients shot snowballs at snowmen and igloos by aiming with their gaze and pressing the spacebar on a keyboard. The snowballs exploded with animations and 3-D sound effects on impact (see Figure 1). Each patient participated in the VR condition, during which they performed active assisted physical therapy exercises. The occupational therapist held the patient’s injured limb (e.g., arm), and the therapist moved the patients limb through a pre-determined sequence of ranging exercises while the patient was in virtual reality (e.g., raising the patients arm as if they were asking a question, or crossing the injured arm across the patient’s chest).
Each patient participated in the control condition, during which they performed active assisted physical therapy exercises with no distraction for the same amount of time they spent doing therapy in VR.
Results.
Alpha values. For each physical therapy session, alpha for t-tests on the 5 pain ratings was conservatively set at p < .01, using a Bonferroni correction factor for multiple comparisons36 to reduce family-wise error (.05/5 comparisons = .01). The number of patients remaining in the study after day three fell from 7 patients to 4 patients, precluding the use of statistics for days 4, 5, 6 or 7. Patients did not lose interest in participating. They either were discharged or required more surgery, precluding additional sessions.
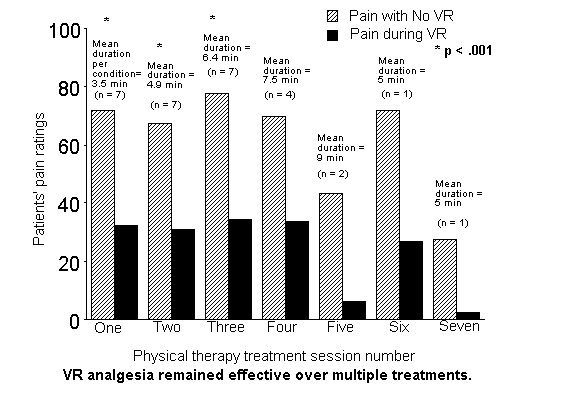
Ratings of pain experienced during treatment. On each day, patients rated pain during physical therapy on five 100 mm visual analog scales (VAS) for each condition (once after therapy in VR, and once after therapy with no VR). As shown in Table 1, and Figure 2, mean VAS pain ratings (Time spent thinking about pain + Unpleasantness + Bothersomeness + Worst pain + Average pain)/5 were significantly higher in the control condition (no distraction) than during VR on each of the first three days. A within-subject ANOVA comparing the “no VR – VR” difference scores from days 1, 2, and 3, showed no difference in the size of the VR analgesia effect F(2,8) < 1, NS. Pain reduction from virtual reality was evident on each of the 5 pain measures, in each of the sessions (see Tables 2, 3 and 4), and did not diminish with repeated use over the first three sessions. Pain reduction is evident even for patients reporting severe to excruciating pain levels during physical therapy. On a zero to 100 mm visual analog scale, six of the seven patients in the present study had mean pain ratings of 70 mm (i.e. severe37,38) or higher during physical therapy with no distraction. And all patients with (and without) severe pain showed VR analgesia.
Descriptive statistics for range of motion, duration of physical therapy, nausea, presence and realism of virtual objects are shown in Table 5. Range of motion was higher in VR than with “no VR” for all seven sessions except session 2. In session 2, range of motion was higher with “no VR” than in VR.
Discussion.
Results from the present study show that VR reduced the amount of pain reported on three separate physical therapy sessions. To our knowledge, this is the first multi-patient study to test whether VR analgesia remains effective when used more than once.
Prior to this study, patients participating in studies on VR analgesia had been trying immersive virtual reality for the first time. Patients in all earlier multi-patient VR analgesia studies received only one VR treatment lasting only 3 minutes per condition (i.e., 3 minutes in VR and 3 minutes with no VR). In the present study, VR was used repeatedly and for over six minutes per condition on day three, with no decline in analgesic potency. VR reduced patients’ pain scores for sensory pain (ratings of worst pain and average pain), as well as affective pain (ratings of unpleasantness and bothersomeness).
Demand Characteristics. Although a standardized treatment protocol
was used, the treating therapist was aware of the treatment condition in the present
study, and this knowledge could potentially have influenced the therapist to
treat the patient differently. One occupational therapist had used VR a number
of times prior to the study, and likely expected it to work based on previous
experience. The other therapist had no
previous experience with VR and likely had no initial pre-disposition to
believe it worked or not. Encouraging
in this regard is the finding that the maximum range of limb motion the patient
could stretch his/her arm was greater in the VR compared to the no-distraction
control condition on six of the seven sessions, suggesting that the therapists
treated patients the same in both the VR and control conditions (as instructed
by the experimenters). The
range-of-motion data suggest that therapists did not “let up” in the intensity
of exercise during VR.
Although more difficult to implement in a clinical setting, double blind experiments are needed to further reduce the likelihood of a demand characteristics explanation of VR analgesia. In a double blind study, at the time the data is collected, neither the experimenter nor the patient knows what the predicted response is for any given experimental condition. Such studies are needed before virtual reality can become a viable form of nonpharmacologic analgesia in everyday medical practice. Patients with severe burns often require dozens of painful physical therapy and wound care procedures lasting approximately 30 to 60 minutes a day during the course of their recovery. Future studies should further increase the frequency and duration of VR treatment, perhaps expanding the number of virtual worlds used by each patient. Such studies should use larger sample sizes since the small sample size used in the present study limits the generalizability of our findings.
Placebo effects. Placebo effects can strongly influence pain
perception in some patients. Beecher’s classic study found that about 35% of
the patients tested experienced pain relief from severe pain such as
post-surgical pain the first time they received a placebo (Beecher, 1959, cited
by Melzack32). Subsequent
studies (described by Melzack32) have found that sugar placebos
became less effective each time they were administered. The fact that only one-third of the patients
responded in Beecher’s study, and that the placebo-based analgesia diminished
each time, dramatically reduces the practical value of using placebos in
everyday medical practice. In contrast
to what would be expected if VR was operating solely through a placebo mechanism,
in the present study the effectiveness of VR analgesia did not diminish
with repeated VR treatments and all seven patients showed VR analgesia. Similarly, a recent study27 found
that over 75% of the burn patients participating showed VR analgesia, a
percentage much higher than would be expected from a placebo effect. Thus VR
analgesia appears to have properties that could have practical medical value as
an adjunctive analgesic. Future
research using a double blind experimental design could greatly reduce the
likelihood that a placebo effect is contributing to VR analgesia. Understanding the mechanism(s) by which VR
analgesia is achieved will likely help us build virtual worlds and VR systems
that maximize the technique’s analgesic effectiveness.
The use of VR-based pain control need not be limited to burn patients. Burn injuries and their treatment are considered to be among the most painful a person can endure. Thus, techniques that prove effective with this population will likely prove effective for other painful procedures (e.g., pain from brief painful cancer procedures, medical procedures requiring the patient to remain conscious or for which repeated sedation is undesirable, physical therapy for cerebral palsy, stroke victims, recovery from knee injuries, etc). Indeed, a case study recently showed that VR appears to be effective with dental pain39. The utility of this technology for controlling chronic pain has yet to be determined. Because of VR’s potential, and the need for new non-pharmacologic adjuncts, additional research on the potential value of VR analgesia during physical therapy is warranted.
Acknowledgements. The Paul Allen Foundation for Medical Research,
NIH grant HD37684-01A1, and NIDRR grant #H133A970014 . Thanks to Mark E. Jensen for valuable
comments. Special thanks to the
University of Washington Burn Staff, the patients, and to Ross Chambers for
generous volunteer fundraising.
References
1. Ward, R.S. Physical Rehabilitation. In Gretchen J. Carrougher Ed., Burn Care and Therapy. 1998, 293-327.
2. Ehde, D.M., Patterson-D.R., and Fordyce, W.E. (1998). The quota system in burn rehabilitation. J-Burn-Care-Rehabil, 19, 436-40.
3. Cooper, J.O. and Pavlin, E.G. Altered pharmacology in thermal injury. Critical Care Report 1990;2:78-84.
4. Patterson, D.R., and Sharar, S.R. Burn pain. In Loeser, J.D, Turk, D., Chapman, C.R., and Butler, S. (Eds.): The Management of Pain, 3rd ed. Philadelphia, Lea & Febiger, 2001, pp.780-787.
5. Patterson, D.R. Non-opioid-based approaches to burn pain. J Burn Care Rehabil, 1995:16:372-6.
6. Cherny N, Ripamonti C, Pereira J, Davis C, Fallon M, McQuay H, Mercadante S, Pasternak G, Ventafridda V. Strategies to manage the adverse effects of oral morphine: an evidence-based report. J Clin Oncol 2001 May 1;19(9):2542-54.
7. Ward RM. Opioid tolerance to sedation and analgesia. Pediatr Res 2000 Jun;47(6):705-6.
8. Brown C, Albrecht R, Pettit H, McFadden T, Schermer C. Opioid and benzodiazepine withdrawal syndrome in adult burn patients. Am Surg 2000 Apr;66(4):367-70.
9. Perry, S., Heidrich, G., and Ramos, E. Assessment of pain by burn patients. J Burn Care Rehabil 1981:2;322-327.
10. Choiniere, M., Melzack, R., Rondeau, J., Girard, N., and Paquin, M.J. The pain of burns: characteristics and correlates. J Trauma 1989;29:1531-9.
11. Melzack, R. The tragedy of needless pain. Scientific American, 1990; 262:27-33.
12. Patterson, D.R, Everett, J.J., Burns, G.L., and Marvin, J.A. Hypnosis for the treatment of burn pain, J Consult Clin Psychol 1992;60:713-717.
13. Miller, A.C., Hickman, L.C., and Lemasters, G.K. A distraction technique for control of burn pain. J. Burn Care Rehabil,1992;13: 576-80.
14. Knudson-Cooper MS: Relaxation and biofeedback training in the treatment of severely burned children. J Burn Care Rehabil 1981;2:102-110,.
15. Tarnowski KJ, MCGrath ML, Calhoun MB, and Drabman RS: Pediatric burn injury: self versus therapist mediated debridement. J Pediatr Psych 1987;12:567-579.
16. Foertsch CE, O'Hara MW, Stoddard JF, and Kealey GP: Parent participation during burn debridement in relation to behavioral distress. J Burn Care Rehabil 1996; 17:372-377.
17. Bernstein NR: Observations on the use of hypnosis with burned children on a pediatric ward. Int J Clin Exp Hypn 1965;13:1-9.
18. Patterson DR, Questad KA, and Boltwood M: Hypnosis applied to pain control in burn patients: a review. J Burn Care Rehabil 1987;8:263-268.
19. Patterson, D., Ptacek, J.T., Carrougher, G. and Sharar, S. (1997). Lorazepam as an adjunct to opioid analgesics in the treatment of burn pain. Pain;72:367-374.
20. Fernandez E, and Turk DC. The utility of cognitive coping strategies for altering pain perception: a meta-analysis. Pain 1989;38:123-135.
21. Hoffman HG, Doctor JN, Patterson DR, Carrougher GJ, and Furness TA III. Use of virtual reality for adjunctive treatment of adolescent burn pain during wound care: A case report. Pain, 2000;85, 305-309.
22. Shiffrin RM, and Schneider W: Controlled and automatic human information processing: II. perceptual learning, automatic attending, and a general theory. Psych Rev 1977;84:127-190.
23. Schneider W, and Shiffrin RM: Controlled and automatic human information processing: I. detection, search, and attention. Psych Rev 1977;84:1-63.
24. McCaul, KD, and Malott, JM. Distraction and coping with pain. Psychological Bulletin 1984;95:516-533.
25. Hoffman, H.G., Prothero, J., Wells, M. and Groen, J. Virtual Chess: The role of meaning in the sensation of presence. International Journal of Human-Computer Interaction, 1998;10:251-263.
26. Patterson, D.R. Practical applications of psychological techniques in controlling burn pain. J. Burn Care Rehabil., 1992;13:13-18.
27. Hoffman HG, Patterson DR, and Carrougher GJ. Use of virtual reality for adjunctive treatment of adult burn pain during physical therapy: a controlled study. Clin J Pain, 2000;16(3):244-50.
28. Hoffman, H.G., Patterson, D.R., Carrougher, G.J., Nakamura, D., Moore, M., Garcia-Palacios, A., and Furness, T.A. III (in press). The effectiveness of Virtual Reality pain control with multiple treatments of longer durations: A case study. International Journal of Human-Computer Interaction.
29. Gift, A.G. Visual analogue scales: measurement of subjective phenomena. Nurs Res 1989;38,286-288.
30. Huskisson, D.C. Measurement of pain, Lancet, 1974;2:1127-31.
31. Melzack, R., and Wall, P.D. Pain Mechanisms: A new theory. Science, 1965;150:971-979.
32. Melzack, R. Psychological Aspects of Pain: Implications for Neural Blockade, in (Cousins, M.J, & Bridenbaugh, P.O., Eds) Neural Blockade: In clinical anesthesia and management of pain, 1998:781-792.
33. Hendrix, C., and Barfield, W. Presence in virtual environments as a function of visual and auditory cues. Proceedings of VRAIS '95, Research Triangle Park, NC. IEEE Computer. Society Press. Los Alamitos, CA, 74-82, 1995.
34. Carlin, A.S., Hoffman, H.G., and Weghorst, S. (1997). Virtual reality and tactile augmentation in the treatment of spider phobia: A case study. Behaviour Research and Therapy, 35, 153-158.
35. Hoffman, H.G., Holander, A., Schroder, K., Rousseau, S. and Furness, T.A., III (1998). Physically touching and tasting virtual objects enhances the realism of virtual experiences. Virtual Reality: Research, Development and Application, 3, 226-234.
36. Keppel G. Design and Analysis: A Researcher’s Handbook 1982; Prentice-Hall, NJ.
37. Jensen, M. P., Smith, D. G., Ehde, D. M., Robinson, L. R. (in press). Pain site and the effects of amputation pain: Further clarification of the meaning of mild, moderate, and severe pain. Pain.
38. Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain 1995; 61: 277-84.
39. Hoffman, H.G., Garcia-Palacios, A., Patterson, D.R., Jensen, M.E., and Furness, T.A.,III (submitted). The effectiveness of Virtual Reality for dental pain control: A case study.
Table 1.
|
Mean VAS = Mean of 5 pain ratings given in each session |
Mean VAS (mm) no distraction |
Mean VAS (mm) VR |
t(6) value |
p value |
SE (difference) |
|
Day 1 |
71.94 |
32.40 |
11.92 |
.000 |
3.08 |
|
Day 2 |
67.41 |
30.84 |
4.53 |
.004 |
8.08 |
|
Day 3 |
77.89 |
34.41 |
4.69 |
.003 |
8.07 |
Table 2, Day 1 pain ratings
|
|
Mean VAS (mm) no distraction |
Mean VAS (mm) VR |
t(6) value |
p value |
SE (differ- ence) |
|
Day1 time spent thinking about pain |
88.71 |
28.57 |
7.08 |
< .001 |
8.50 |
|
Day 1 unpleasant |
58.00 |
31.14 |
4.65 |
.003 |
5.77 |
|
Day 1 bothersome |
72.43 |
34.86 |
2.98 |
.025 |
12.62 |
|
Day 1 worst pain |
76.43 |
39.14 |
3.04 |
.023 |
12.25 |
|
Day 1 average pain |
69.00 |
28.29 |
t(5)= 3.37 |
.020 |
10.66 |
Table 3, Day 2 pain ratings
|
|
Mean VAS (mm) no distraction |
Mean VAS (mm) VR |
t(6) value |
p value |
SE (differ- ence) |
|
Day 2 time spent thinking about pain |
67.57 |
23.86 |
4.25 |
.005 |
10.28 |
|
Day 2 unpleasant |
62.71 |
30.14 |
3.39 |
.015 |
9.61 |
|
Day 2 bothersome |
67.43 |
34.86 |
2.80 |
.031 |
11.65 |
|
Day 2 worst pain |
80.14 |
37.43 |
3.67 |
.010 |
11.63 |
|
Day 2 average pain |
68.40 |
33.60 |
t(4)= 4.62 |
.010 |
7.53 |
Table 4, Day 3 pain ratings
|
|
Mean VAS (mm) no distraction |
Mean VAS (mm) VR |
t(6) value |
p value |
SE (differ- ence) |
|
Day 3 time spent thinking about pain |
73.00 |
23.86 |
4.54 |
.004 |
10.82 |
|
Day 3 unpleasant |
72.29 |
29.29 |
3.30 |
.016 |
13.04 |
|
Day 3 bothersome |
77.71 |
34.29 |
4.87 |
.003 |
8.92 |
|
Day 3 worst pain |
78.00 |
52.43 |
2.68 |
.036 |
9.53 |
|
Day 3 average pain |
53.50 |
31.00 |
t(4)= 4.32 |
.012 |
7.69 |
Table 5.
Day 1 Day 2 Day 3 Day 4 Day 5 Day 6 Day 7
(n = 7) (n = 7) (n = 7) ( n = 4) (n = 2) (n = 1) (n = 1)

Figure
1. An image from SnowWorld.
Figure 2
Appendix A. Information presented to patients at time of recruitment.
Hi, my name is Gretchen Carrougher and I am a research nurse here at Harborview in the Burn Center. Your nurse (or doctor) asked you if it was okay for me to talk with you about our research and I understand that that is okay. There is a research study that you can participate if you wish -- in other words, you don't have to if you don't want too – The study
concerns pain control during physical therapy. We are doing a study on the use of VR for pain control during physical therapy. You would perform your physical therapy just like you always do, except you will go into virtual reality for a few minutes while you are doing your physical therapy. Then we will stop and you will answer a few questions about how much pain you experienced. Then we will do more physical therapy for the same amount of time without virtual reality, after which you will answer the same pain ratings again to assess how much pain you experienced.
Would you be interested in participating in this study?